About Vacuum Technology
Vacuum is a space that has all matter removed.
- The number of molecules per cc in the average earth’s atmosphere is approximately 2.5 X 1019 or 25,000,000,000,000,000,000. By comparison, the average number of molecules per cc in Intergalactic Space is 3.
- A manufactured vacuum considered high order in a dewar contains approximately 3.3 X 1011 or 330,000,000,000 molecules per cc.
- The molecules in the earth’s atmosphere randomly move at about 1,000 MPH at ambient atmospheric temperature.
- Ambient atmospheric pressure at sea level is approximately 15 psig or 760 torr.
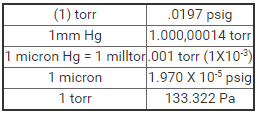
At room temperature and normal atmospheric pressure, one cubic foot (0.03 cubic meter) of air contains approximately 7 x 1023 molecules moving in random directions and at speeds of around 1,000 miles per hour (1,600 kilometers per hour). The momentum exchange imparted to the walls equals a force of 14.7 pounds for every square inch of wall area. This atmospheric pressure can be expressed in several different units, but until recently, it was commonly expressed in terms of the weight of a column of mercury of unit cross section and 760 millimeters (mm) high. Thus, one standard atmosphere equals 760 mm Hg, but to avoid the anomaly of equating different units, a term, torr, has been postulated. One standard atmosphere = 760 torr (1 torr = 1 mm Hg). This term was replaced in 1971 by the SI unit defined as the Newton per square meter (N/m2) and called the Pascal (one Pascal = 7.5×10-3 torr).
Vacuum technology is an essential element of cryogenic systems. A high-order vacuum (10-5torr) in a vacuum-insulated vessel is required for low heat transfer at cryogenic temperatures. The gas molecules are so far apart at these low pressures that convection is virtually eliminated. Under this pressure level, the molecules strike the sides of their channel more often than they cross each other. The gas, under this condition, is not treated as a continuous medium with the free molecular flow.
Vacuum order is typically regarded as follows:
- Rough Vacuum – down to 25 torr
- Medium Vacuum – 25 torr down to 1 X 10-3 torr
- High Order Vacuum – 1 X 10-3 torr down to 1 X 10-6 torr
- Very High Order Vacuum – 1 X 10-6 torr down to 1 X 10-9 torr
- Ultra High order Vacuum – below 1 X 10-9 torr
(All values at cryogenic temperatures)
Typically, a high-order vacuum is created by a series of vacuum pumps removing gas molecules sequentially. Mechanical roughing pumps can operate down to about 1 X 10-3 torr. These can be rotary vane or rotary piston pumps. Adding a Roots-style rotary pump to the inlet of the roughing pump can lower the pressure into the 1 X 10-4 range. This addition also substantially reduces oil back streaming and contamination.
The diffusion pump can operate effectively from about 1 X 10-2 down to about 1 X 10-7torr. The turbomolecular pump typically operates from 1 X 10-2 down to 1 X 10-7. Vac-ion pumps range from 1 X 10-4 down to 1 X 10-9 torr. The cryopump can be used in a range from about 1 X 10-3 down to 1 X 10-10.
History of Vacuum
Evangelista Torricelli was a physicist and mathematician who invented the barometer. In 1643 he filled a four-foot-long glass tube with mercury and inverted the tube into a dish. He observed that some of the mercury did not flow out and that above the mercury in the tube was a vacuum. Torricelli became the first man to create a sustained vacuum. After much observation, he concluded that the variation of the height of the mercury from day to day was caused by changes in the atmospheric pressure.
Robert Boyle developed Boyle’s Law. At Oxford he collaborated with Robert Hooke in constructing an air pump with which he conducted experiments leading to his publication (1662) of the relationship that at constant temperature the volume of a gas is inversely proportional to its pressure
Amedeo Avogadro developed Avogadro’s Law. Under the same conditions of temperature and pressure, equal volumes of different gasses contain an equal number of molecules. This empirical relation, proposed by the Italian physicist Amadeo Avogadro in 1811, can be derived from the kinetic theory of gases under the assumption of a perfect (ideal) gas. The law is approximately valid for real gases at sufficiently low pressures and high temperatures.
The specific number of molecules is given by Avogadro’s constant. In the meter-kilogram-second (mks) system of units, Avogadro’s constant is the number of molecules in one kilogram-mole of a substance, defined as the molecular weight in kilograms. The value of Avogadro’s constant is 6.023 X 1026 molecules per kilogram-mole in mks units or 6.023 X 1023 molecules per gram-mole in the centimeter-gram-second (cgs) system of units.
For example, the molecular weight of oxygen is 32, so that one kilogram-mole of oxygen has a mass of 32 kilograms and contains 6.023 X 1026 molecules.
The volume occupied by one mole of gas is about 22.4 liters at standard temperature and pressure (STP), which are 0°C and 1 atmosphere pressure, and is the same for all gases according to Avogadro’s law.
Applications of Vacuum
The first major use of vacuum technology in industry occurred about 1900 in the manufacture of electric light bulbs. Other devices requiring a vacuum for their operation followed, such as various types of electron tube. Furthermore, it was discovered that certain processes carried out in a vacuum achieved either superior results or ends actually unattainable under normal atmospheric conditions. Such developments included the “blooming” of lens surfaces to increase the light transmission, the preparation of blood plasma for blood banks, and the production of reactive metals such as titanium. The advent of nuclear energy in the 1950s provided impetus for development of vacuum equipment on a large scale. Increasing applications for vacuum processes were steadily discovered, as in space simulation and microelectronics.
Uses for Vacuum
Industrial vacuum applications range from mechanical handling (such as the manipulation of heavy and light items by suction pads) to the deposition of integrated electronic circuits on silicon chips. Obviously, vacuum requirements are as widely varied as the particular processes using vacuums. In the rough vacuum range from about one torr to near atmosphere, typical applications are mechanical handling, vacuum packing and forming, gas sampling, filtration, degassing of oils, concentration of aqueous solutions, and impregnation of electrical components, distillation, and steel stream degassing.
Chemical processes and Vacuum
At lower pressures down to about 10-4 torr, many metallurgical processes such as melting, casting, sintering, heat treatment, and brazing can derive benefit. Chemical processes such as vacuum distillation and freeze-drying also need this range of vacuum. Freeze-drying is used extensively in the pharmaceutical industry to prepare vaccines and antibiotics and to store skin and blood plasma. The food industry freeze-dries coffee mainly, although most foods can be stored without refrigeration after freeze-drying, and the technique is receiving widespread acceptance.
The pressure range down to about 10-6 torr is used for cryogenic (low-temperature) and electrical insulation. It is used in the production of lamps; television picture tubes, X-ray tubes; decorative, optical, and electrical thin-film coatings; and mass spectrometer leak detectors.
Thin-film coating
In thin-film coating, a metal or compound is evaporated under high vacuum from a source onto a base material or substrate. The base material is generally plastic for decorative coatings; glass for optical coatings; and glass ceramic, or silica for electrical coatings. Thickness of the film can vary from about 1/4 wavelength of visible light to 0.001 inches or more. In the optical field, antireflection coatings are deposited on lenses for cameras, telescopes, eyeglasses, and other optical devices, considerably reducing the amount of light reflected by the lenses and thus giving a brighter transmitted image.
To achieve vacuum high enough for thin-film coating and for other industrial uses requiring pressures down to 10-6 torr, a pumping system consisting of an oil-sealed rotary pump and a diffusion pump is used. The oil-sealed rotary pump (sometimes referred to as fore pump) “roughs” the chamber down to a pressure of about 0.1 torr, after which the roughing valve is closed. The fore valve and high-vacuum baffle valve are then opened so that the chamber is evacuated by the diffusion pump and rotary pump in series.
Vacuum Equipment
Oil-sealed Rotary Pump
Capacities are available from 1/2 to 1,000 cubic feet per minute, operating from atmospheric pressure down to as low as 2 X 10-2 torr for single-stage pumps and less than 5 X 10-3 torr for two-stage pumps. The pumps develop their full speed in the range from atmosphere to about one torr. The speed then decreases to zero at their ultimate pressures. Two of the most common designs are useful for pumping both liquids and gases. One is a two-bladed pump in which the rotor is eccentric to the stator, forming a crescent-shaped volume swept by the blades through the outlet valve. The second, a rotary piston pump, similar to a single blade, is part of the sleeve fitting around the rotor. The blade is hollow and acts as an inlet valve, closing off the pump from the system when the rotor is at top center.
Ultimate pressures attainable are limited by leakage between the high- and low-pressure sides of the pump (due mainly to carry over of gases and vapors dissolved in the sealing oil that flash off when exposed to the low inlet pressure) and decomposition of the oil exposed to high temperature spots generated by friction.
Gas ballasting helps to prolong pump life because it removes the chief source of pump contamination, condensable vapors. The gas ballast is a vented exhaust that admits a small amount of air at atmospheric pressure to the compression side of the pump, thus permitting most condensable vapors to pass through the pump without condensing.
Typical applications of this pump are in food packaging, high-speed centrifuges, and ultraviolet spectrometers. It is also widely used as a fore pump or a roughing pump, or both, for most of the other pumps described.
Mechanical Booster
Capacities are available from 100 to 70,000 cubic feet per minute, operating usually in the pressure range from 10 to 10-3 torr. The peak speed of the pump is developed in the pressure range from 1 to 10-2 torr. The speed at the lower end of the pressure range depends on the type of fore pump used. A typical mechanical booster uses two figure-eight-shaped impellers, synchronized by external gears, rotating in opposite directions inside the stator. The gas is trapped from between the impellers and the stator wall and transferred from the high vacuum to the fore vacuum side of the pump. The gears are oil-lubricated but are external to the pump, so that the impellers run dry.
Clearance between the impellers and the stator wall is generally about .002 to .010-inch. As a consequence, back leaking of gas occurs at a rate governed by the pressure difference between input and output and the type of gas being pumped. Under normal running conditions, a pressure difference of about ten to one is obtained. The mechanical booster must be backed by another pump in series when working in its normal pressure range. The most frequently used type of fore pump is the oil-sealed rotary pump. Typically, the mechanical booster is employed for pumping vacuum-melting furnaces, in an impregnation plant for electrical equipment, and in low-density wind tunnels.
Molecular Pump
Capacities are available up to 20,000 cubic feet per minute, with an operating range of 10 -1 to 10-10torr, when backed by an oil-sealed rotary pump. The full speed of the pump is developed in the very wide pressure range from 10-2 to 10-9 torr. In the molecular pump, a high rotational speed rotor (up to 32,000 revolutions per minute) imparts momentum to the gas molecules, moving them along the small clearance between the rotor and stator. The molecular drag pump also employs this principle of operation. For ultimate base pressures it has been almost entirely displaced by the faster and simpler turbo molecular pump, in which radial slots in both rotor and stator fins actuate the pump.
A number of compression stages are employed but because of its design, larger clearances can be tolerated between rotor and stator than were possible in the molecular drag pump. The molecular drag pump is sometimes built integral with a turbo molecular pump to allow the use of very clean “dry” backing pumps. These hybrids are often used in semiconductor processing where oil vapor back streaming would contaminate processes but high pumping speeds are needed.
Vapor Diffusion Pump
This pump is mainly used on equipment for the study of clean surfaces and in radio frequency sputtering. Pumping speeds are available up to 190,000 cubic feet per minute with an operating pressure range of 10-2 to less than 10-9 torr when water-cooled baffles are used and less than 10-11torr when refrigerated baffles are employed. The pumping speed for a vapor pump remains constant from about 10-3 torr to well below the ultimate pressure limitations of the pump fluid. The best fluids allow pressures of better than 10-9 torr. The diffusion pump is initially evacuated by an oil-sealed rotary pump to a pressure of about 0.1 torr or less. When the pump fluid in the boiler is heated, it generates a boiler pressure of a few torr within the jet assembly. High-velocity vapor streams emerge from the jet assembly, impinge and condense on the water or air-cooled pump walls, and return to the boiler. In normal operation part of any gas arriving at the inlet jet is entrained, compressed, and transferred to the next stage. This process is repeated until the gas is removed by the mechanical fore pump.
The oil-vapor booster pump works on the same principles as the diffusion pump, but it employs a higher boiler pressure. Normal operating pressure range is 1 to 10-4 torr. When backed by an oil-sealed rotary pump, this pump is widely used for achieving high vacuum in thin-film evaporation units, accelerators, and in TV tube pumping.
Sputter Ion Pump
Capacities are available up to 14,000 cubic feet per minute, with an operating pressure range of 10-11 torr. The full speed of the pump is developed in the pressure range from about 10-6 to 10-8 torr, although the characteristic at the lower pressure is dependent on the pump design. This pump uses a cathode material such as titanium vaporized or sputtered by bombardment with high velocity ions. The active gasses are pumped by chemical combination with the sputtered titanium, the inert gasses by ionization and burial in the cathode, and the light gasses by diffusion into the cathode.
A typical pump consists of two flat rectangular cathodes with a stainless steel anode between them made up of many open-ended boxes. This assembly, mounted inside a narrow box attached to the vacuum system, is surrounded by a permanent magnet. The anode is operated at a potential of about seven kilovolts (kV), whereas the cathodes are at ground potential.
The sputter ion pump has low speeds and sometimes instability when pumping inert gases. To improve its characteristics, other types of sputter ion pumps have been developed: the slotted cathode, triode, differential, and magnetron pumps.
To start up a sputter ion pump it is necessary to reduce the pressure to at least 2 X 10-2 torr, and preferably much lower, by means of a roughing pump. Sputter ion pumps can operate in any position and do not need water or liquid nitrogen supplies. They have a long life and can provide very clean, ultrahigh vacuum, free of organic contamination and vibration. They are employed mainly for the clean-surface studies and in those applications where any organic contamination will give unsatisfactory results.
Cryopump
A vacuum cryopump typically consists of a vacuum-tight vessel with a valved inlet, containing a highly absorbent material such as a synthetic zeolite microporous on the scale of 0.1 to 1 nanometers, and an enclosing cryogenic vessel. In industrial cryopumps, the expansion of compressed helium is used to cool a cold head, so the process is continuous. Although the gas is trapped in the cryopump throughout normal operation, the cryopump can remain cold for months or even years in normal high and ultrahigh vacuum operation. At some point, the pump is shut down, and allowed to heat up. The trapped gasses evaporate and are flushed out, a process knows as regeneration. Since cryopumps don’t use any oil in the vacuum side, they are used when very clean pumping is needed.
Sorption pumps are a type of cryopump that is often used as roughing pumps to reduce pressures from the range of atmospheric to on the order of 10-3 torr (0.1 Pa), while lower pressures are achieved using a finishing pump. As the sorbent saturates, the effectiveness of a sorption pump decreases, but can be recharged by heating the zeolite material (preferably under conditions of low pressure) to a temperature near but below the breakdown point of the zeolite material’s porous structure.
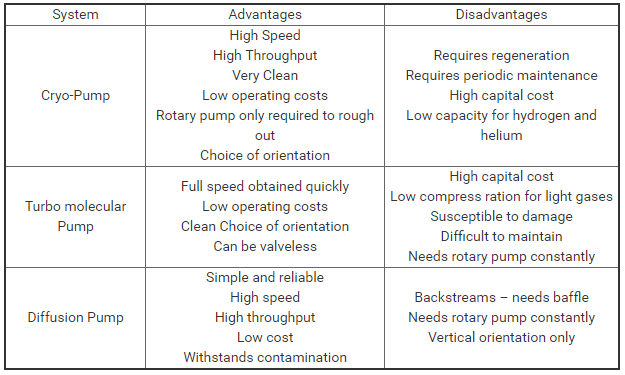
Advantages and disadvantages of each vacuum pumping system:
Vacuum Research
Almost every research laboratory uses vacuum directly in its experiments or employs equipment that depends on vacuum for its operation. The lowest pressures are obtained in the research laboratories, where equipment is generally similar to, but smaller than that used by industry.
Typical of the research equipment using vacuum down to about 10-6 torr are the electron microscope, analytical mass spectrometer, particle accelerator, and large space simulation equipment. Particle accelerators range from small van de Graaff machines to large proton synchrotrons.
In space simulation, large units that simulate space around a complete vehicle require a vacuum of 10-6 torr or below. Such vessels incorporate a complete shroud at liquid nitrogen temperature and a port through which high-intensity light can be beamed to simulate the sun’s radiation.
In the pressure region down to and below 10-9 torr, research applications include electrical insulation, thermonuclear energy conversion experiments, microwave tubes, field ion microscopes, field emission microscopes, storage rings for particle accelerators, specialized space simulator experiments, and clean-surface studies. In many experiments it is not only necessary to reach such pressures of 10-9 torr but to reduce the hydrocarbons in the residual gases to an absolute minimum. Even small traces of hydrocarbons can render the results unreliable. To achieve a vacuum of this order the vacuum vessel and the equipment inside must be cleared of residual gas (degassed) to the greatest extent possible. A common solution is to bake the whole apparatus for a number of hours at about 350°C while maintaining a vacuum in the 10-5 torr region. Baking at this temperature requires the use of all-metal sealing rings. To eliminate hydrocarbons, the unit is pumped down to about 10-3 torr using sorption pumps; and from there, sputter ion pumps and titanium sublimation pumps complete the task down to 10-9 torr or below.
References: www.mcallister.com/vacuum.html

